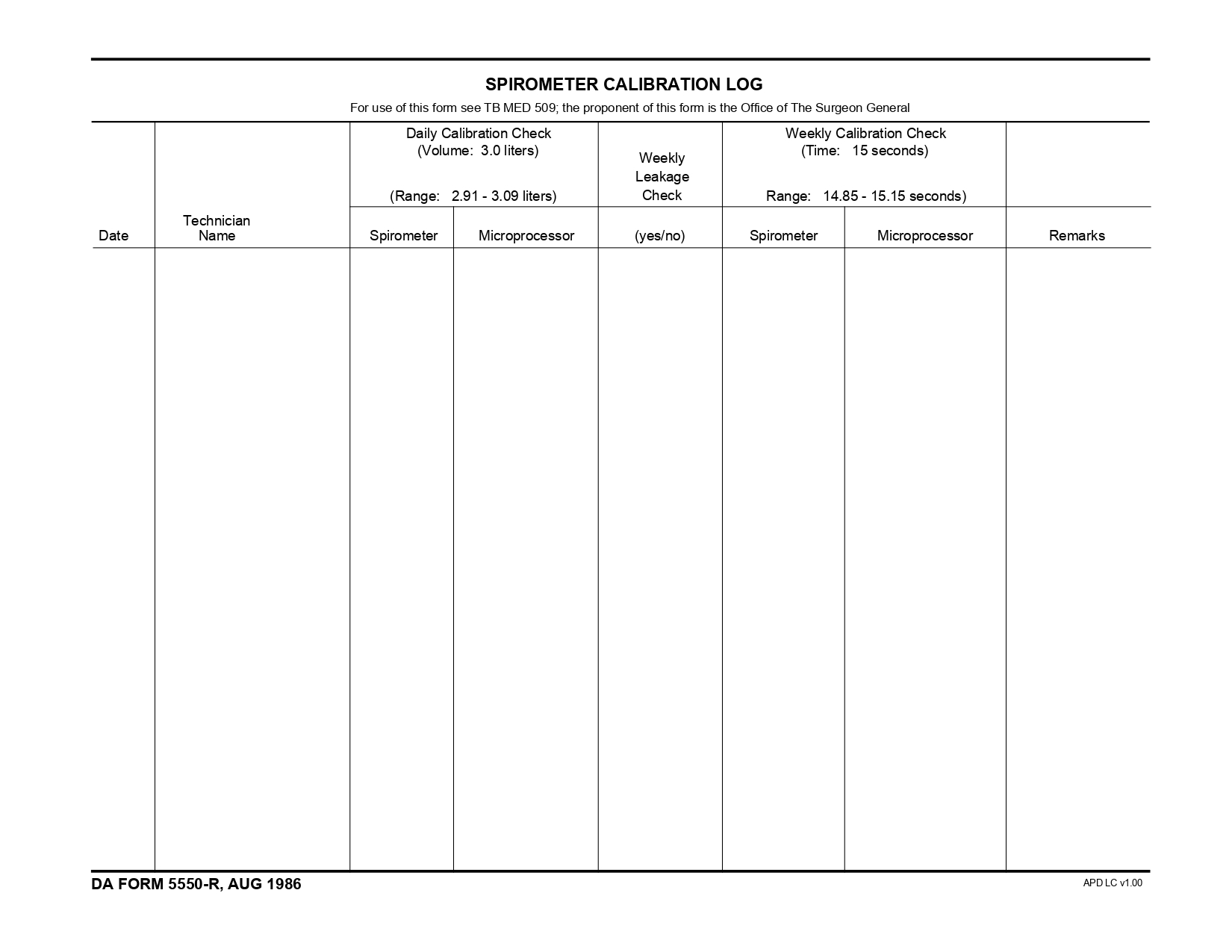
DAFORMFILLABLE.COM | DA FORM 5550-R Fillable – Army Pubs 5550-R PDF – DA FORM 5550-R, also known as the Spirometer Calibration Log (LRA), is a crucial document used in the medical field, specifically for recording the calibration of spirometers. Spirometers are vital instruments used to measure lung function, essential for diagnosing various respiratory conditions. Maintaining an accurate calibration log ensures that the readings obtained from these devices are reliable and accurate.
DA FORM 5550-R – Spirometer Calibration Log (LRA)
| Form Number | DA Form 5550-R |
| Form Title | Spirometer Calibration Log (LRA) |
| Form Date | 08/01/1986 |
| Form Proponent | TSG |
Overview of DA FORM 5550-R
Publication Details
- Pub/Form Number: DA FORM 5550-R
- Pub/Form Date: 08/01/1986
- Pub/Form Title: Spirometer Calibration Log (LRA)
- Pub/Form Proponent: TSG (The Surgeon General)
- Pub/Form Status: ACTIVE
- Prescribed Forms/Prescribing Directive: TB MED 509
- Footnotes: Also produced in electronic media
- Security Classification: UNCLASSIFIED
- Distribution Restriction Code: A (Approved for public release; distribution is unlimited)
- Pub/Form IDN: 990001
- Pub/Form PIN: 060823
Importance of Spirometer Calibration
Ensuring Accurate Measurements
Calibration of spirometers is essential for obtaining accurate measurements of lung function. Regular calibration, recorded in the Spirometer Calibration Log (LRA), helps in maintaining the integrity of the device, ensuring that the results are consistent and reliable.
Compliance with Medical Standards
Recording calibration details on DA FORM 5550-R helps healthcare facilities comply with medical standards and regulations. This compliance is critical for patient safety and the accuracy of medical diagnoses.
Using DA FORM 5550-R
Structure of the Form
The Spirometer Calibration Log (LRA) is designed to capture detailed information about each calibration session. Key sections of the form include:
- Date of Calibration: The specific date when the calibration was performed.
- Calibration Results: Detailed results obtained from the calibration process.
- Technician Information: Name and signature of the technician performing the calibration.
- Remarks: Any additional notes or observations relevant to the calibration session.
Recording and Storing Calibration Data
Properly recording calibration data on DA FORM 5550-R involves documenting each calibration session meticulously. This log should be stored securely and be easily accessible for audits and inspections.
Compliance and Distribution
TB MED 509
TB MED 509 prescribes the use of DA FORM 5550-R. This technical bulletin outlines the procedures and standards for spirometer calibration, ensuring that all healthcare facilities adhere to the same high standards of practice.
Public Release and Accessibility
The form is classified as UNCLASSIFIED and carries a distribution restriction code of A, meaning it is approved for public release and its distribution is unlimited. This wide availability ensures that all relevant parties can access and utilize the form as needed.
Conclusion
The DA FORM 5550-R – Spirometer Calibration Log (LRA) is an essential tool for maintaining the accuracy and reliability of spirometers in medical settings. By adhering to the guidelines outlined in TB MED 509 and properly recording calibration data, healthcare providers can ensure the highest standards of patient care and diagnostic accuracy. Regular calibration and meticulous record-keeping are not only best practices but also critical components of respiratory health management.
DA FORM 5550-R Fillable – Army Pubs 5550-R PDF DOWNLOAD