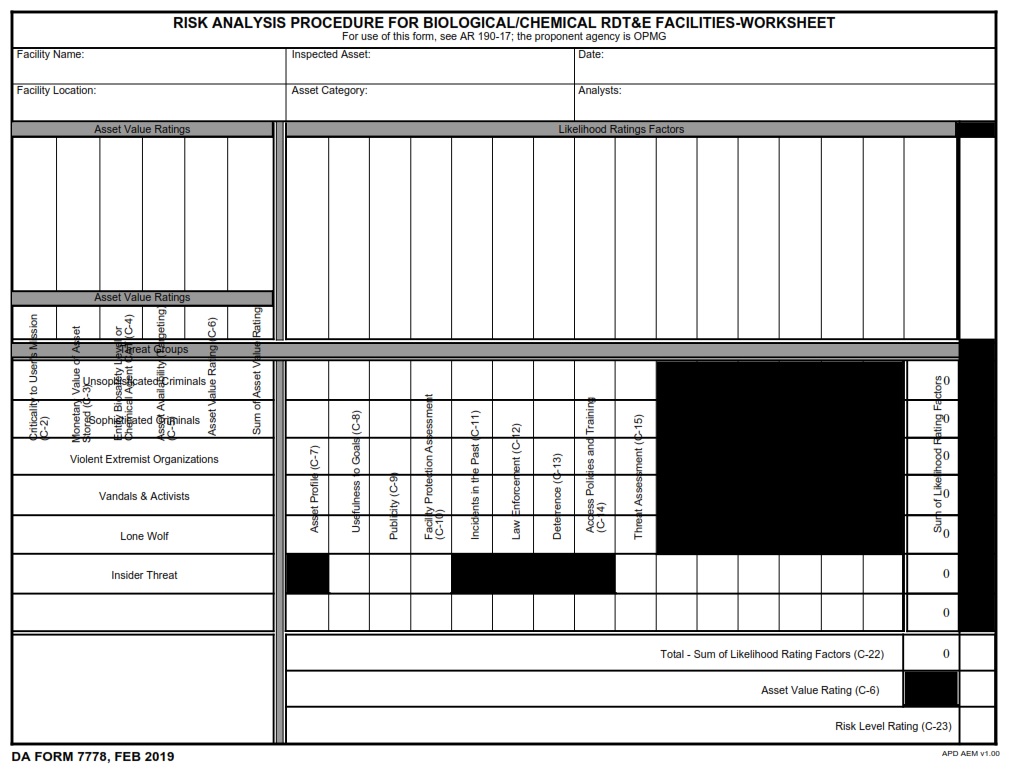
DAFORMFILLABLE.COM | DA FORM 7778 Fillable – Army Pubs 7778 PDF – DA FORM 7778 is a crucial document used for the Risk Analysis Procedure for Biological/Chemical Research, Development, Test, and Evaluation (RDT&E) Facilities. Issued on February 1, 2019, this form is an essential tool for ensuring the safety and security of facilities dealing with biological and chemical materials.
DA FORM 7778 – Risk Analysis Procedure For Biological/Chemical RDT&E Facilities – Worksheet
| Form Number | DA FORM 7778 |
| Form Title | Risk Analysis Procedure For Biological/Chemical RDT&E Facilities – Worksheet |
| Form Date | 2/1/2019 |
| Form Proponent | PMG |
Purpose of DA FORM 7778
The primary purpose of DA FORM 7778 is to provide a structured method for analyzing risks associated with biological and chemical RDT&E activities. This form helps facility managers and safety officers identify potential hazards, assess risks, and implement necessary controls to mitigate those risks.
Key Sections of DA FORM 7778
1. Identification and Description
This section captures basic information about the facility and the specific biological or chemical materials being used. It includes details such as the location, the nature of the RDT&E activities, and the personnel involved.
2. Risk Assessment
Here, the form guides users through a systematic process to evaluate the potential risks. This involves:
- Identifying Hazards: Recognizing all possible sources of danger within the facility.
- Assessing Exposure: Determining who might be exposed to these hazards and under what circumstances.
- Evaluating Consequences: Considering the potential impact of these hazards if they were to occur.
3. Risk Control Measures
This section focuses on identifying and implementing controls to mitigate the identified risks. Controls can include engineering solutions, administrative policies, personal protective equipment, and emergency response plans.
The Importance of Risk Analysis in Biological/Chemical RDT&E Facilities
Risk analysis is vital in facilities dealing with biological and chemical materials due to the high stakes involved. Proper risk management ensures:
- Safety of Personnel: Protecting the health and well-being of researchers and staff.
- Security of Materials: Preventing unauthorized access and potential misuse of dangerous substances.
- Environmental Protection: Minimizing the risk of accidental releases that could harm the environment.
Regulatory Compliance and Prescribing Directive
DA FORM 7778 is prescribed by AR 190-17, which outlines the requirements for security and risk management in biological and chemical RDT&E facilities. Compliance with these regulations is mandatory to maintain the operational integrity and safety of these facilities.
Accessibility and Distribution
The form is classified as UNCLASSIFIED and falls under Distribution Restriction Code A, meaning it is approved for public release and distribution is unlimited. This ensures that all relevant stakeholders can access and utilize the form as needed.
Conclusion
DA FORM 7778 serves as an indispensable tool for risk analysis in biological and chemical RDT&E facilities. By following the structured procedure outlined in the form, facilities can effectively identify, assess, and control risks, thereby ensuring the safety and security of their operations. This form not only facilitates regulatory compliance but also promotes a culture of safety and responsibility in handling hazardous materials.